Molecular Modeling With Avogadro. This is a simple and fun, hands-on activity that demonstrates the basic concepts of computer-based molecular modeling. Using molecular mechanics, small molecules may be modeled by treating the atoms as balls and the bonds connected them as springs. 1.1.1: Describe the mole concept and apply it to substances. The mole concept applies to all kinds of particles: atoms, molecules, ions, formula units etc. The amount of substance is measured in units of moles. The approximate value of Avogadro's constant (L), 6.02 x 10 23 mol-1, should be known.
Science >Chemistry >Molecule and Molecular Mass > Mole Concept

In this article, we are going to study very important concept of chemistry known as the mole concept.
Berzelius Hypothesis:
According to Dalton’s atomic theory atoms of different elements combine with each other in a simple whole number ratio, whereas Gay Lussac’s law of combining volumes, gases combine with each other in a simple whole ratio by their volumes.
A Swedish chemist Berzelius correlated the two laws and put forward is the concept called Berzelius hypothesis. It states that equal volumes of all gases under similar conditions of temperature and pressure contain an equal number of atoms.
An Italian chemist Avogadro in 1811, modified the theory by differentiating between the ultimate particle of an element that takes part in reaction (atom) and the ultimate particle that has independent existence (molecule). He argued that the smallest particle of a substance which has independent existence is not an atom but molecule.
Avogadro’s Law:
Equal volumes of all gasses under the same conditions of temperature & pressure contain an equal number of molecules.
Explanation:
Let two gasses gas A and Gas B be taken in two containers having equal volume (V). Let the temperature of both the gasses be the same (T).Let the pressure of the two gases be same (P). By Avogadro’s law under such conditions of equal pressure, equal volume & equal temperature, the number of molecules of gas A in the container should be equal to the number of molecules of gas B in the container.
Validation of Avogadro’s Law:
Consider following chemical reactions
H2 + Cl2 → 2HCl
1vol 1vol 2 vol
Thus the simple ratio of volumes is 1 : 1 : 2
Suppose 1 volume of hydrogen gas contains ‘n’ molecules. By Avogadro’s law at same temperature and pressure, there should be ‘n’ molecules of chlorine and ‘2n’ molecules of hydrogen chloride are involve.
Thus ‘n’ molecules of H2 + ‘n’ molecules of Cl2 → ‘2n’ molecules of HCl
Dividing this equation by n, we have
1 molecule of H2 + 1 molecule of Cl2 → 2 molecules of HCl
Hydrogen and chlorine are diatomic molecules.
2 atoms of H2 + 2 atoms of Cl2 → 2 molecules of HCl
Dividing this equation by 2, we have
1 atom of H2 + 1 atom of Cl2 → 1 molecule of HCl
Now, number atoms of hydrogen, chlorine and hydrogen chloride are the whole numbers the Avogadro’s law is validated.
Importance of Avogadro’s Law:
- It differentiates between atoms and molecules of gasses.
- It modified Dalton’s atomic theory.
- It explains Gay Lussac’s law of combining volume.
- It helps in determination of the atomic weight of elements.
- It established that at N.T.P.one gram mole of any gas occupies 22.4 dm3 by volume. one mole of a gas contains 6.023 × 1023 molecules of gas.
- It gives the relation between the vapour density & the molecular mass. The relation is
Molecular weight = 2 × vapour density.
Concept of Vapour Density:
The vapour density of a gas is defined as the ratio of the weight of a certain volume of a gas to the weight of the same volume of hydrogen at the same temperature & pressure.
Relation Between Molecular Mass and vapour Density:

The vapour density of a gas is defined as the ratio of the weight of a certain volume of a gas to the weight of the same volume of hydrogen at the same temperature & pressure. By definition of vapour density
This is the relation between the molecular weight and vapour density
Gram Molecular Volume (Molar volume) (GMV):
The volume occupied by one mole of a gas at STP (or NTP) is called as gram molar volume. It is 22.4 dm3 at NTP.
Proof:
Mathematically, Avogadro’s law is stated as “At constant temperature (T) and pressure (P) the volume (V) of a gas is directly proportional to the number of molecules (n).
The molecular mass of gas corresponds to one mole of a gas hence we can say that one mole of a gas occupies 22.4 dm3 at STP.
Atomicity:
The number of atoms present in a molecule of a substance is called the atomicity of that substance.
Examples: The number in bracket indicates atomicity of that compound. Helium He (1), Dioxygen O2 (2), Ozone O3 (3), 4 Phosphorous P4 (4), Sulphur S8 (8), Carbon dioxide CO2 (3), Ammonia NH3 (4).
Atomicity indicates that how many atoms are present in the molecule of the element or the compound.
Atomicity of Element using Avogadro’s Law:
The number of molecules present in a molecule of a substance is called the atomicity of substance.
Let us consider the formation of ammonia. Experimentally it is found that 1 volume of nitrogen reacts with 3 volumes of hydrogen to form 2 volumes of ammonia. Thus,
N2 + 3H2 → 2NH3
1 vol 3 vol 2 vol

1/2 vol 3/2 vol 1vol
According to Avogadro’s law “equal volumes of all gases under the identical condition of temperature and pressure contain the same number of molecules”. If 1 volume of ammonia contains n molecules 1/2 volume of nitrogen would contain n /2 molecules and 3/2 volume of hydrogen would contain 3n/2 molecules.
∴ n/2 molecules of Nitrogen + 3n/2 molecules of Hydrogen → n molecules of Ammonia
∴ 1/2 molecule of nitrogen + 3/2 molecule of hydrogen → 1molecule of ammonia.
As a molecule can be divided into the constituent atoms but the atoms cannot be divided further nitrogen molecule has to be diatomic, hydrogen diatomic and ammonia tetra-atomic.
Thus atomicity of Nitrogen =2, atomicity of hydrogen = 2 and atomicity of ammonia = 4.
Mole Concept:
The term mole was introduced by Wilhelm Ostwald. In Latin mole means heap or pile. He assumed substance as heap or pile of elementary entities like atoms, molecules, ions, etc. and called it as a mole.
When we are studying matter we require the actual number of molecules involved in the reaction. The atoms and molecules are discrete particles present in large number in the matter. To denote this number of atoms or molecules the term mole is used.
The amount of substance that contains as many elementary entities, like atoms, molecules, ions, photons as there are atoms in 0.012 kg of carbon 12 is called mole of a substance.
Example: 1 mole of hydrogen means 2 g of hydrogen. 1 mole of oxygen means 32 g of oxygen.
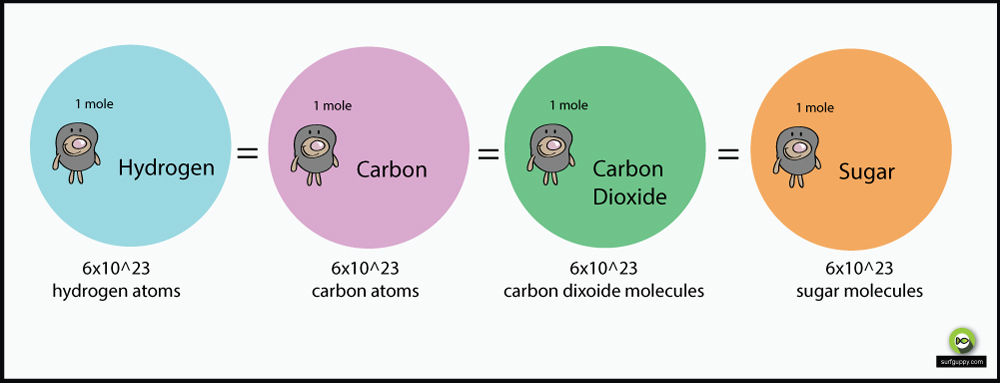
- A mole is a fundamental unit of the amount of substance in the SI system of units. It is a collection of 6.022 × 1023 particles. The number 6.022 × 1023 is also called Avogadro’s number.
- 1 mole also corresponds to the atomic or molecular mass of an element expressed in gram. Thus 1 mole of gas contains 6.022 × 1023 molecules of gas. A 1-mole atom of gas contains 6.022 × 1023 atoms of gas. 1-mole ions contain 6.022 × 1023 ions etc.
Mole and Avogadro ’s Number:
The number of particles such as atoms, molecules, ions, in one mole of a substance is called Avogadro’s number or Avogadro’s constant.
It is denoted by ‘N’. Its value at STP is 6.022 × 1023 per mole. Thus 1 mole of a gas contains 6.022 × 1023 molecules of gas. 1-mole atom of a gas contains 6.022 × 1023 atoms of gas. 1-mole ions contain 6.022 × 1023 ions etc.
The Significance of Avogadro’s Number:
- Avogadro’s number is equal to the number of molecules in one gram mole or one gram molecular mass of any compound. Thus gram molecular mass of any substance is equal to the mass in grams of Avogadro’s number of 6.022 × 1023 molecules.
- Avogadro’s number is equal to the number of atoms in one gram mole or one gram atomic mass of an element. Thus gram atomic mass of any element is equal to the weight in grams of Avogadro’s number of 6.022 × 1023 atoms.
- Avogadro’s number is equal to the number of molecules in 22.4 dm3 of any gas at NTP.
- The actual mass of an element or compound can be found using this number.
Concept of one gram atom:
One gram atom of an element is the amount of the element which is equal to the mass of 1 mole (or 6.023 × 1023 atoms) of the element or atomic mass of the element in grams.
Thus one gram atom of sodium is equal to one mole of sodium corresponds to 23 grams of sodium, because the atomic mass of sodium is 23 grams.
Science >Chemistry >Molecule and Molecular Mass > Mole Concept
Science StandardsExplore the discoverer's biography, including general facts about his life and anecdotes regarding how he made this particular discovery. Also see other significant scientific discoveries built largely on this concept and other real-world applications in history that may not still be relevant.
Discoverer/Developer
Avogadro's Law from 1811. Amedeo Avogadro (1776-1856) was an Italian physicist and lawyer. He taught physics in Turin, Italy. Avogadro published two memoirs, once in 1811, and the second in 1814. Both were published in French. Avogadro's Law was stated separately by Ampere in 1814, and the two are generally interchangeable, historically speaking.
Use/Application through History
Avogadro's Law is widely regarded as a significant contributor to the determination of atomic and molecular weights. Gaudin and Cannizzaro used Avogadro's law to determine atomic weights. Avogadro suggested the diatomic nature of many 'elementary' gases, as did Dumas using Avogadro's Law. The Law also contributed to the determination of molecular formulae by demonstrating the ratios of combinations of gases, such as in the gases of water, hydrogen chloride, ammonia, and nitric oxide. Cannizzaro also used Avogadro's Law to 'simplify the teaching of chemistry' and present a unified system consistent for chemical and physical observations. Avogadro's Law contributed to the development of the kinetic theory of matter. Avogadro's Law can also be applied to osmosis, which, in turn, allows the determination of molecular weights by osmotic pressure.
Study the primary definition of this concept, broken into general, basic, and advanced English definitions. Also see the mathematical definition and any requisite background information, such as conditions or previous definitions.
General Science
Volume increases as the number of particles increases if temperature and pressure stay constant. Volume decreases as the number of particles decreases if temperature and pressure stay constant.
Basic
Volume and the number of particles of a gas are directly proportionate for a constant temperature and pressure.
Advanced
The ratio of volume (V) and the number of particles (n) of a gas is a constant. Volume and the number of particles of a gas are directly proportionate for a constant temperature and pressure.
Background Information
Constante De Avogadro Concepto
Ideal Gas
An 'ideal gas' is a gas in which:
- All collisions are totally elastic (particles always bounce off each other)
- There are no intermolecular attractions (a particle can only change direction when it collides with another particle)
- The molecule is infinitely small (particles will come all the way together before they collide)
What does this mean? An ideal gas is a collection of bouncy-balls.
Avogadro Concept
Discover processes or disciplines in the natural or man-made worlds that employ the concept.
Avogadro's Law, along with other gas laws, explains why bread and other baked goods rise. Yeast or other leavening agents in the dough break down the long carbohydrates from the flour or sugar and convert them into carbon dioxide gas and ethanol. The carbon dioxide forms bubbles, and, as the yeast continues to leaven the dough, the increase in the number of particles of carbon dioxide increase the volume of the bubbles, thereby puffing up the dough.
Avogadro's Law explains projectiles, like cannons and guns; the rapid reaction of the gunpowder very suddenly creates a large amount of gas particles--mostly carbon dioxide and nitrogen gases--which increase the volume of the space behind the cannon or bullet until the projectile has enough speed to leave the barrel.
A balloon inflates because of Avogadro's Law; the person blowing into the balloon is inputing a lot of gas particles, so the balloon increases in volume.
Avogadro's Law Concept
We breathe because of Avogadro's Law, among others; the lungs expand, so more gas particles can enter the lungs from the outside air (inhaling). Then the lungs contract, so the waste gas particles are expelled (exhaling).
Learn important vocabulary for this concept, including words that might appear in assessments (tests, quizzes, homework, etc.) that indicate the use of this concept.
| Term | Context |
| ', CAPTION, 'evolve',ABOVE,CENTER, WIDTH, 200, FGCOLOR, '#50EFFF', BGCOLOR, '#2298DF', TEXTCOLOR, '#000000', CAPCOLOR, '#FFFFFF', OFFSETX, 10, OFFSETY, 10, CELLPAD, 5, BORDER, 2);' onmouseout='return nd();'>Evolves |
|
| ', CAPTION, 'make',ABOVE,CENTER, WIDTH, 200, FGCOLOR, '#50EFFF', BGCOLOR, '#2298DF', TEXTCOLOR, '#000000', CAPCOLOR, '#FFFFFF', OFFSETX, 10, OFFSETY, 10, CELLPAD, 5, BORDER, 2);' onmouseout='return nd();'>Makes |
|
| ', CAPTION, 'particle',ABOVE,CENTER, WIDTH, 200, FGCOLOR, '#50EFFF', BGCOLOR, '#2298DF', TEXTCOLOR, '#000000', CAPCOLOR, '#FFFFFF', OFFSETX, 10, OFFSETY, 10, CELLPAD, 5, BORDER, 2);' onmouseout='return nd();'>Particles |
|
| ', CAPTION, 'produce',ABOVE,CENTER, WIDTH, 200, FGCOLOR, '#50EFFF', BGCOLOR, '#2298DF', TEXTCOLOR, '#000000', CAPCOLOR, '#FFFFFF', OFFSETX, 10, OFFSETY, 10, CELLPAD, 5, BORDER, 2);' onmouseout='return nd();'>Produces |
|
| ', CAPTION, 'yield',ABOVE,CENTER, WIDTH, 200, FGCOLOR, '#50EFFF', BGCOLOR, '#2298DF', TEXTCOLOR, '#000000', CAPCOLOR, '#FFFFFF', OFFSETX, 10, OFFSETY, 10, CELLPAD, 5, BORDER, 2);' onmouseout='return nd();'>Yields |
|
Experience computer simulators or animations that illustrate the concept discussed here. Many simulators or animations come with worksheets for use in class.
| http://www.grc.nasa.gov/WWW/K-12/airplane/Animation/frglab2.html |
| http://phet.colorado.edu/simulations/sims.php?sim=Gas_Properties |
| http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/gasesv6.swf |
| http://intro.chem.okstate.edu/1314F00/Laboratory/GLP.htm |
| http://preparatorychemistry.com/Bishop_animations.htm |

Read a summary of the concept, indicating the enduring understanding students should retain after class.
If the pressure and temperature remain constant, increasing the number of particles of gas inside a container will increase the volume of the container. Likewise, decreasing the number of particles of gas inside a container will decrease the volume of the container.
