Atomic Mass of Hydrogen Atomic mass of Hydrogen is 1.0079 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.
- Hydrogen Atomic Mass Is Called
- Hydrogen Atomic Mass Number
- Hydrogen Protons Electrons Neutrons
- Hydrogen Atomic Mass Amu
Learning Objective
- Discuss the chemical properties of hydrogen’s naturally occurring isotopes.
Atomic Mass of Hydrogen Atomic mass of Hydrogen is 1.0079 u. Hydrogen Atom MassBy: Mr. Pradeep Kshetrapal, Tutorials Point India Private Limited.
Key Points
- Protium is the most prevalent hydrogen isotope, with an abundance of 99.98%. It consists of one proton and one electron. It is typically not found in its monoatomic form, but bonded with itself (H2) or other elements.
- Deuterium is a hydrogen isotope consisting of one proton, one neutron and one electron. It has major applications in nuclear magnetic resonance studies.
- Tritium is a hydrogen isotope consisting of one proton, two neutrons and one electron. It is radioactive, with a half-life of 12.32 years.
Terms
- diatomicConsisting of two atoms.
- isotopeForms of an element where the atoms have a different number of neutrons within their nuclei. As a consequence, atoms of the same isotope will have the same atomic number, but a different mass number.
Properties of Isotopes of Hydrogen
Hydrogen has three naturally occurring isotopes: 1H (protium), 2H (deuterium), and 3H (tritium). Other highly unstable nuclei (4H to 7H) have been synthesized in the laboratory, but do not occur in nature. The most stable radioisotope of hydrogen is tritium, with a half-life of 12.32 years. All heavier isotopes are synthetic and have a half-life less than a zeptosecond (10-21 sec). Of these, 5H is the most stable, and the least stable isotope is 7H .
Protium
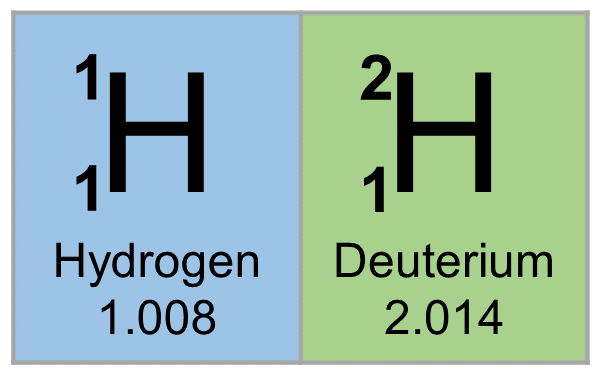
1H is the most common hydrogen isotope with an abundance of more than 99.98%. The nucleus of this isotope consists of only a single proton (atomic number = mass number = 1) and its mass is 1.007825 amu. Hydrogen is generally found as diatomic hydrogen gas H2, or it combines with other atoms in compounds—monoatomic hydrogen is rare. The H–H bond is one of the strongest bonds in nature, with a bond dissociation enthalpy of 435.88 kJ/mol at 298 K. As a consequence, H2 dissociates to only a minor extent until higher temperatures are reached. At 3000K, the degree of dissociation is only 7.85%. Hydrogen atoms are so reactive that they combine with almost all elements.
Deuterium
2H, or deuterium (D), is the other stable isotope of hydrogen. It has a natural abundance of ~156.25 ppm in the oceans, and accounts for approximately 0.0156% of all hydrogen found on earth. The nucleus of deuterium, called a deuteron, contains one proton and one neutron (mass number = 2), whereas the far more common hydrogen isotope, protium, has no neutrons in the nucleus. Because of the extra neutron present in the nucleus, deuterium is roughly twice the mass of protium (deuterium has a mass of 2.014102 amu, compared to the mean hydrogen atomic mass of 1.007947 amu). Deuterium occurs in trace amounts naturally as deuterium gas, written 2H2 or D2, but is most commonly found in the universe bonded with a protium 1H atom, forming a gas called hydrogen deuteride (HD or 1H2H).
Chemically, deuterium behaves similarly to ordinary hydrogen (protium), but there are differences in bond energy and length for compounds of heavy hydrogen isotopes, which are larger than the isotopic differences in any other element. Bonds involving deuterium and tritium are somewhat stronger than the corresponding bonds in protium, and these differences are enough to make significant changes in biological reactions. Deuterium can replace the normal hydrogen in water molecules to form heavy water (D2O), which is about 10.6% denser than normal water. Heavy water is slightly toxic in eukaryotic animals, with 25% substitution of the body water causing cell division problems and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure). Consumption of heavy water does not pose a health threat to humans. It is estimated that a 70 kg person might drink 4.8 liters of heavy water without serious consequences.
The most common use for deuterium is in nuclear resonance spectroscopy. As nuclear magnetic resonance (NMR) requires compounds of interest to be dissolved in solution, the solution signal should not register in the analysis. As NMR analyzes the nuclear spins of hydrogen atoms, the different nuclear spin property of deuterium is not ‘seen’ by the NMR instrument, making deuterated solvents highly desirable due to the lack of solvent-signal interference.
Tritium
3H is known as tritium and contains one proton and two neutrons in its nucleus (mass number = 3). It is radioactive, decaying into helium-3 through beta-decay accompanied by a release of 18.6 keV of energy. It has a half-life of 12.32 years. Naturally occurring tritium is extremely rare on Earth, where trace amounts are formed by the interaction of the atmosphere with cosmic rays.
Hydrogen Atomic Mass Is Called
Heavier Synthetic Isotopes
4H contains one proton and three neutrons in its nucleus. It is a highly unstable isotope of hydrogen. It has been synthesized in the laboratory by bombarding tritium with fast-moving deuterium nuclei. In this experiment, the tritium nuclei captured neutrons from the fast-moving deuterium nucleus. The presence of the hydrogen-4 was deduced by detecting the emitted protons. Its atomic mass is 4.02781 ± 0.00011 amu. It decays through neutron emission with a half-life of 1.39 ×10−22 seconds.
5H is another highly unstable heavy isotope of hydrogen. The nucleus consists of a proton and four neutrons. It has been synthesized in a laboratory by bombarding tritium with fast-moving tritium nuclei. One tritium nucleus captures two neutrons from the other, becoming a nucleus with one proton and four neutrons. The remaining proton may be detected and the existence of hydrogen-5 deduced. It decays through double neutron emission and has a half-life of at least 9.1 × 10−22 seconds.
6H decays through triple neutron emission and has a half-life of 2.90×10−22 seconds. It consists of one proton and five neutrons.
7H consists of one proton and six neutrons. It was first synthesized in 2003 by a group of Russian, Japanese and French scientists at RIKEN’s RI Beam Science Laboratory, by bombarding hydrogen with helium-8 atoms. The helium-8’s neutrons were donated to the hydrogen’s nucleus. The two remaining protons were detected by the “RIKEN telescope”, a device composed of several layers of sensors, positioned behind the target of the RI Beam cyclotron.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
Hydrogen Atomic Mass Number
http://en.wiktionary.org/wiki/isotope
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Hydrogen_isotopes
Wikipedia
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/diatomic
Wiktionary
CC BY-SA 3.0.
https://commons.wikimedia.org/wiki/File:Protium_deuterium_tritium.jpg
Wikimedia Commons
CC BY-SA 3.0.
http://en.wikipedia.org/w/index.php?title=File:Hydrogen.svg&page=1
Wikipedia
CC BY-SA.
So then, why isn't the atomic mass of Hydrogen exactly 1?
If you check a periodic table, you'll see that Hydrogen actually has a mass of 1.00794. If hydrogen is the lightest of all substances, then why not give it a mass of exactly 1 on our relative mass scale?
There are three reasons:
- First, atoms have isotopes, and these isotopes do not all have the same mass. The mass of the atoms in nature - what we use as the atomic mass - is a weighted average of all these different isotopes.
Here are the exact atomic masses and abundances of an atom with two imaginary stable isotopes.
To 4 significant digits, what would be the calculated atomic mass of naturally occurring X? |
- The second reason is historical. Once upon a time, way back before 1961, there actually were two sets of atomic masses (though everybody called them atomic weights then). One scale was used by physicists; the other by chemists. Both were based on weights compared to Oxygen, rather than Hydrogen. Oxygen was used because it combines with a lot of things to form oxides. This made it a better choice as a standard because of the ease of chemical analysis. Oxygen was set to have an atomic mass of 16, which was just about 16 times as heavy as Hydrogen being 1. Unfortunately, Chemists picked naturally occurring Oxygen, which is a mixture of isotopes of Oxygen-16, Oxygen-17, and Oxygen-18. After all when you made an oxide of an element you would do so in naturally occurring oxygen. Physicists picked the pure isotope Oxygen-16, because they tended to make their measurements on the basis of mass spectrometry.
Though the ratio of any two atom's masses was the same on either scale, it was horribly confusing, so in 1961, a compromise was reached. Instead of using either Hydrogen, or Oxygen as the standard, the isotope of Carbon with 6 protons and 6 neutrons in its nucleus (Carbon-12) was given a mass of exactly 12. It was a good choice, since it was in between the two previously used standards, and meant that nothing had to change too much.
| Which of the following statements is correct? |
- The third reason is the most important of all. If a hydrogen atom has only one proton, and carbon-12 has 6 protons and 6 neutrons to make up its mass of twelve, why isn't the mass of hydrogen 1/12 of that of carbon-12?
Mass of 1 hydrogen atom Mass of sub-atomic particles Mass of 1 carbon-12 atom 1.00794 6 protons = 6 x 1.007277 6.043662 6 neutrons = 6 x 1.008665 6.051990 6 electrons = 6 x 0.000548 0.003288 Total 12.098940 12.0 exactly If you think about it, Hydrogen at 1.00794 is more than 1/12 of the weight of carbon-12 (as you can see from the above table, if you multiply 12 times the mass of a single hydrogen atom it comes to more than 12). The reason for this effect is nuclear binding energy. After all, the protons in the nucleus are all positive, and so the nucleus should just repel itself apart. It doesn't of course, so something must be 'binding' it together. This nuclear binding energy makes the mass of all atoms (except hydrogen-1, which only has 1 proton) slightly lighter that what you'd get by adding up the mass of the sub-atomic particles. Einstein's famous equation E = mc2 shows us that we can get the necessary binding energy from the mass of the sub-atomic particles. So the mass of any multi-nucleon atom is less than the sum of the weights of its separated parts. Its this change in mass when the nucleus changes size that is the source of the enormous amount of energy in nuclear reactions.
So we could have set hydrogen to be exactly 1, but then we'd have had to really revise the atomic weight table back in 1961. If hydrogen was assigned a mass of 1 exactly, then oxygen would have become 15.87, quite a difference from the mass chemists were using. Choosing carbon-12 as the reference standard meant the least change was necessary. Still, if you do really accurate calculations based on the old and the new scale you can see some differences. For example, on the pre-1961 atomic weight scale the molecular weight of table salt, Sodium chloride NaCl would have been 58.45. On today's scale it is 58.44. The difference is just 0.02%, so for most purposes it wouldn't matter.
Hydrogen Protons Electrons Neutrons
Hydrogen Atomic Mass Amu
Hold it! You just used the term molecular weight. Isn't that wrong? Yes, of course it is, but for Sodium chloride, we shouldn't even use the term molecular mass. Instead we should use the term 'formula mass', because Sodium Chloride really isn't a molecule of NaCl.
Copyright © 1998 - 2008 David Dice
